Objective 8: Public Health and Clinical Data Registry Reporting
Objective: The EP![]() Eligible Professional: a Medicaid provider who qualifies for the Medicaid Promoting Interoperability Program is in active engagement with a public health agency (PHA) or clinical data registry (CDR) to submit electronic public health data in a meaningful way using CEHRT
Eligible Professional: a Medicaid provider who qualifies for the Medicaid Promoting Interoperability Program is in active engagement with a public health agency (PHA) or clinical data registry (CDR) to submit electronic public health data in a meaningful way using CEHRT![]() Certified EHR Technology, an EHR that conforms to the ONC's Health IT Certification Program criteria and standards, except where prohibited, and in accordance with applicable law and practice.
Certified EHR Technology, an EHR that conforms to the ONC's Health IT Certification Program criteria and standards, except where prohibited, and in accordance with applicable law and practice.
| An EP must satisfy two measures for this objective. If the EP cannot satisfy at least two measures, they may take exclusions from all measures they cannot meet. | |
| Measure 1: Immunization Registry Reporting: The EP is in active engagement with a PHA to submit immunization data and receive immunization forecasts and histories from the public health immunization registry/immunization information system (IIS). | |
| Exclusion |
An EP may take an exclusion if any of the following apply:
|
| Reporting | EPs must attest YES to being in active engagement with a PHA to submit immunization data and receive immunization forecasts and histories from the public health immunization registry/immunization information system (IIS). |
| Measure 2: Syndromic Surveillance Reporting: The EP is in active engagement with a PHA to submit syndromic surveillance data. | |
| Exclusion |
An EP may take an exclusion if any of the following apply:
|
| Reporting | EPs must attest YES to being in active engagement with a PHA to submit syndromic surveillance data from an urgent care setting. |
| Measure 3: Electronic Case Reporting: The EP is in active engagement with a PHA to submit case reporting of reportable conditions. | |
| Exclusion |
An EP may take an exclusion if any of the following apply:
|
| Reporting | EPs must attest YES to being in active engagement with a PHA to submit case reporting of reportable conditions. |
| Measure 4: Public Health Registry Reporting: The EP is in active engagement with a PHA to submit data to public health registries. | |
| Exclusion |
An EP may take an exclusion if any of the following apply:
|
| Reporting | EPs must attest YES to being in active engagement with a PHA to submit data to public health registries. |
| Measure 5: Clinical Data Registry Reporting: The EP is in active engagement to submit data to a CDR. | |
| Exclusion |
An EP may take an exclusion if any of the following apply:
|
| Reporting | EPs must attest YES to being in active engagement to submit data to a CDR. |
CMS defines active engagement as the process of moving towards sending production data to a PHA or CDR or sending production data to a PHA or CDR. "Production data" refers to data generated through clinical processes involving patient care, and it is used to distinguish between data and "test data" which may be submitted for the purposes of enrolling in and testing electronic data transfers.
Active engagement may be demonstrated in one of the following ways:
- Option 1 – Completed Registration to Submit Data: The EP registered to submit data with the PHA or, where applicable, the CDR to which the information is being submitted; registration was completed within 60 days after the start of the EHR reporting period; and the EP is awaiting an invitation from the PHA or CDR to begin testing and validation. This option allows EPs to meet the measure when the PHA or the CDR has limited resources to initiate the testing and validation process. EPs that have registered in previous years do not need to submit an additional registration to meet this requirement for each EHR reporting period.
- Option 2 – Testing and Validation: The EP is in the process of testing and validation of the electronic submission of data. EPs must respond to requests from the PHA or, where applicable, the CDR within 30 days; failure to respond twice within an EHR reporting period would result in that EP not meeting the measure.
- Option 3 – Production: The EP eligible clinician has completed testing and validation of the electronic submission and is electronically submitting production data to the PHA or CDR.
EPs interested in exchanging data with a public health immunization registry/immunization information system (IIS) should contact Sevocity Support to begin the process of a new interface setup. Interface setup requirements and fees vary per request.
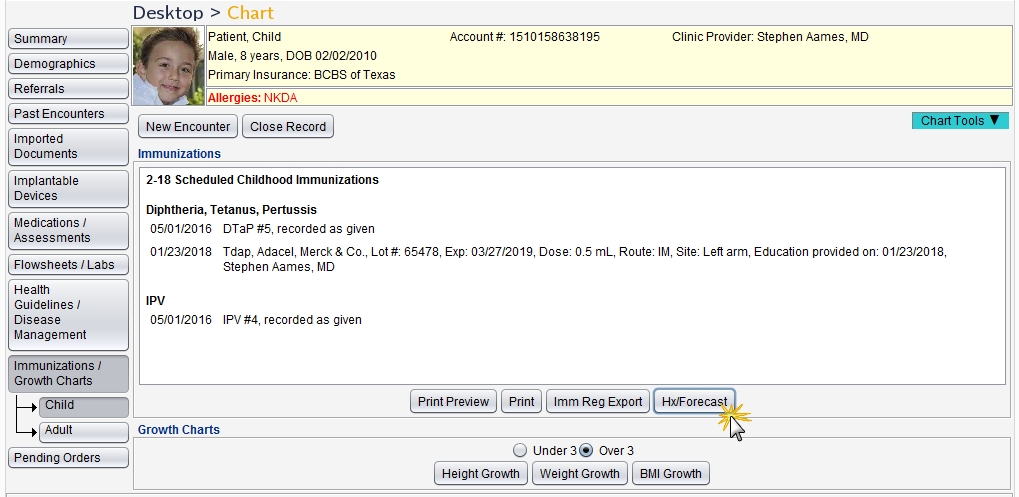
Customers who have an interface with a registry that provides history and forecast information will be able to access a patient’s immunization histories and forecasts from the patient chart or encounter.
- Chart > Immunizations/Growth Charts > Hx/Forecast button
- Encounter > Immunizations > Hx/Forecast button
EPs interested in exchanging data with a public health agency (PHA) should contact Sevocity Support to begin the process of a new interface setup. Interface setup requirements and fees vary per request.
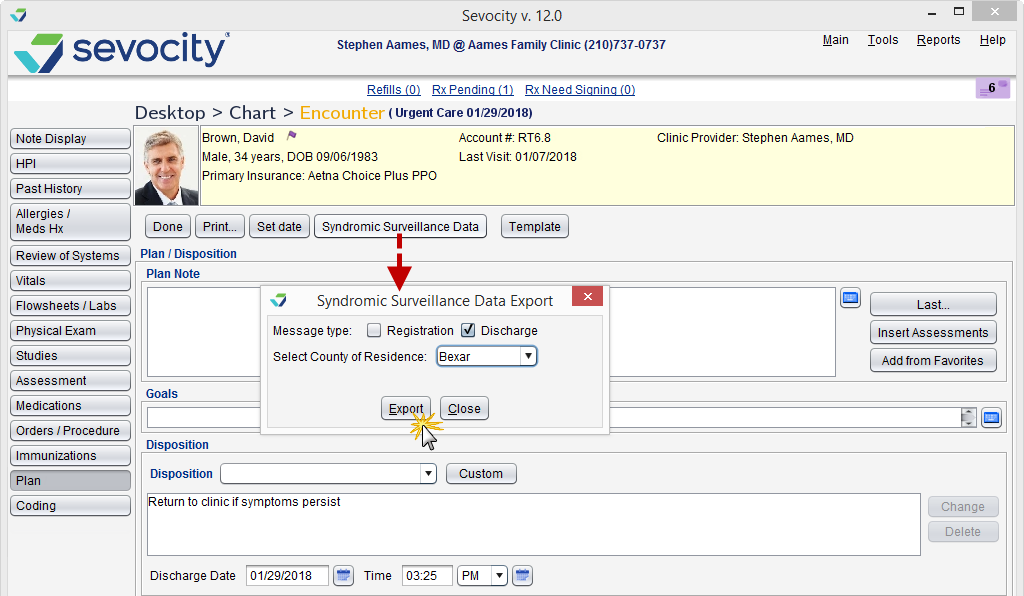
The ability to create a syndromic surveillance data file in Sevocity is available only from the Urgent Care encounter type. Clinic Administrators can enable and disable the Urgent Care encounter type by going to Tools > Preferences > CLINIC User > Encounter Types
- From the Urgent Care encounter, click the Syndromic Surveillance Data button
- Select a message type: Registration or Discharge
- Select the patient’s County of Residence
- Click Export
- Type a name for the file and click Save to save to a local machine
The encounter data is exported as an HL7![]() Health Level-7, a set of international standards for the transfer of data between Health IT systems output file that can be used for submission to a public health agency.
Health Level-7, a set of international standards for the transfer of data between Health IT systems output file that can be used for submission to a public health agency.
EPs interested in exchanging data with a public health agency (PHA) should contact Sevocity Support to begin the process of a new interface setup. Interface setup requirements and fees vary per request.
EPs interested in exchanging data with a clinical data registry (CDR) should contact Sevocity Support to begin the process of a new interface setup. Interface setup requirements and fees vary per request.
- An exclusion for a measure does not count toward the total of two measures. Instead, in order to meet this objective, an EP needs to meet two of the total number of measures available to them. If the EP qualifies for multiple exclusions and the remaining number of available measures is less than two, the EP can meet the objective by meeting all of the remaining available measures and claiming the applicable exclusions. Available measures are ones for which the EP does not qualify for an exclusion.
- EPs who have previously registered, tested, or begun ongoing submission of data to a registry do not need to "restart" the process.
- For Measure 4, EPs may choose to report to more than one public health registry to meet the number of measures required to meet the objective.
- For Measure 5, EPs may choose to report to more than one CDR to meet the number of measures required to meet the objective.
- For Measure 5, the definition of jurisdiction is general, and the scope may be local, state, regional or at the national level. The definition will be dependent on the type of registry to which the EP is reporting. A registry that is "borderless" would be considered a registry at the national level and would be included for purposes of this measure.
Return to 2019 Medicaid Promoting Interoperability Objectives
Didn't find the answer you were looking for?
Contact Sevocity Support 24/7 at 877‑777‑2298 or support@sevocity.com